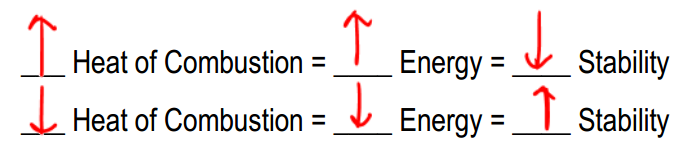
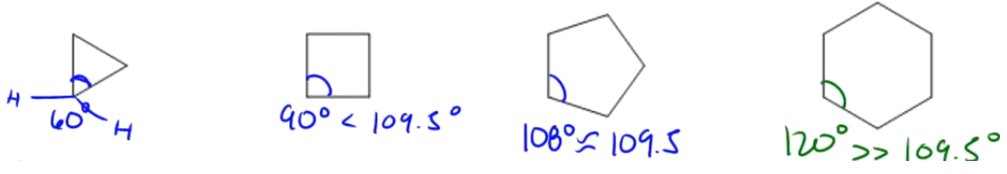
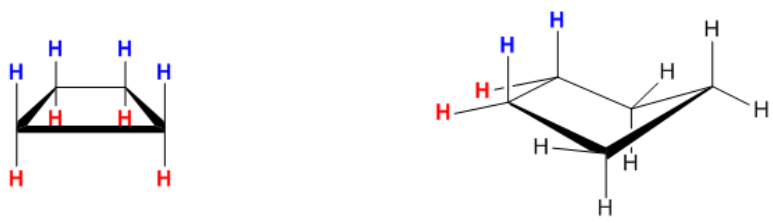
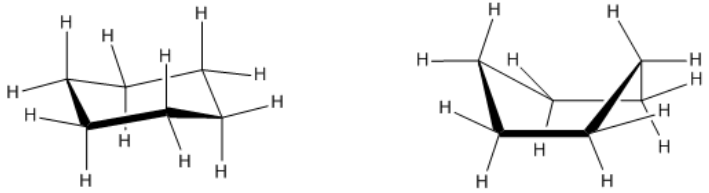
The heat of combustion is an analytical technique used to measure the energy content of a molecule by observing the amount of heat released during its combustion. Essentially, this process involves igniting a substance to determine how much energy is released, which is indicative of the molecule's stability and energy levels. A higher heat of combustion signifies that more energy is released during the reaction, suggesting that the molecule is less stable and possesses higher energy. Conversely, a lower heat of combustion indicates that the molecule is more stable and has lower energy content.
Understanding the relationship between heat of combustion, energy, and stability is crucial. When evaluating molecules, one might encounter terms like "most stable molecule," "most energetic molecule," or "lowest heat of combustion." Recognizing that a high heat of combustion correlates with high energy and low stability, while a low heat of combustion indicates low energy and high stability, is essential for making informed comparisons. Thus, the concepts of energy release and stability are inversely related, providing a framework for analyzing molecular characteristics.