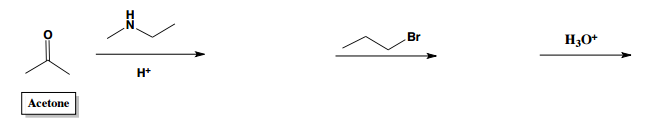
Enamine alkylation and acylation are significant reactions in organic chemistry, particularly involving ketones and aldehydes. When secondary amines react with ketones, they form enamines, which are characterized by a structure that includes an amine group and an alkene. The key feature of enamines is their nucleophilic alpha carbon, which allows them to participate in nucleophilic attacks.
In the process of alkylation or acylation, an enamine reacts with an electrophile, commonly an alkyl halide. The mechanism begins with the lone pair of electrons on the nitrogen atom forming a double bond with the alpha carbon, while simultaneously breaking a bond with the nitrogen. This results in the formation of an iminium salt, where the nitrogen carries a positive charge. This step is crucial as it signifies the successful alkylation of the alpha carbon.
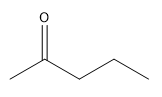
To convert the iminium salt back to a ketone, an acid workup is performed, which involves hydrolysis. This process transforms the nitrogen-containing compound into an oxygen-containing compound, specifically a ketone that is now alpha-substituted. Understanding this transformation is essential, as it highlights the relationship between nitrogen and carbonyl compounds in organic synthesis.
For further practice, it is beneficial to work through examples of these reactions. Start with the first example, applying the concepts discussed, and then review the solution to solidify your understanding. Following that, tackle the second example collaboratively to enhance your grasp of the mechanisms involved.