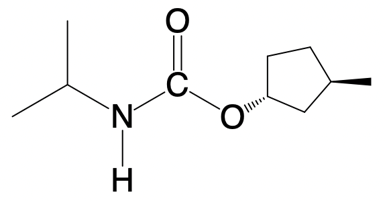
Urethane formation involves the creation of a carbonate ester through a nucleophilic addition reaction. This process occurs when an alcohol reacts with an isocyanate molecule. A carbonate ester is characterized by a carbonyl group (C=O) that is bonded to an amino group (N) and an alkoxyl group (O-R), where R represents a carbon atom or hydrogen.
In the reaction, the isocyanate features a reactive carbon atom that is double-bonded to a nitrogen atom. When the alcohol approaches, the pi bond of the isocyanate is broken, allowing the alcohol to attach. Specifically, the hydrogen atom from the alcohol is transferred to the nitrogen atom, forming an NH bond. Meanwhile, the remaining alkoxyl portion (O-R) of the alcohol bonds to the carbonyl carbon, resulting in the formation of the urethane structure.
This reaction can be summarized as follows: the isocyanate (R-N=C=O) reacts with the alcohol (R'-OH) to yield the urethane (R-NH-C(=O)-O-R'). This simplified explanation highlights the essential components of urethane formation without delving into the detailed reaction mechanism.