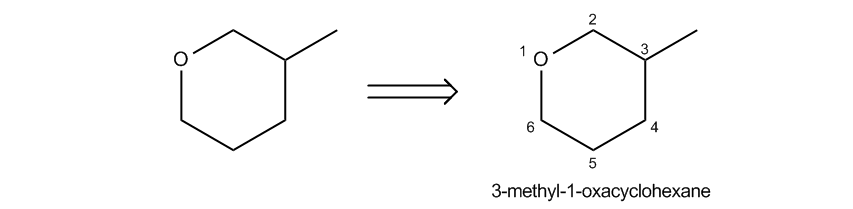
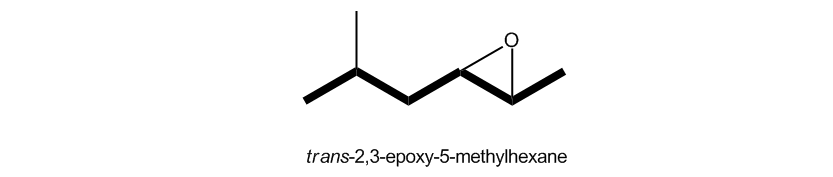
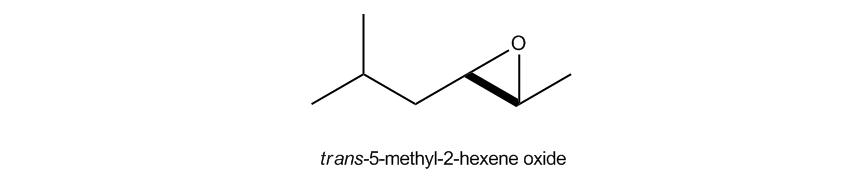
Epoxides, also known as oxeranes, are a specific type of cyclic ether characterized by their three-membered ring structure. This unique configuration leads to significant ring strain, as the bond angles deviate from the typical tetrahedral angle of 109.5 degrees found in most ethers. Due to this strain, epoxides exhibit increased reactivity compared to other ethers, making them notable in organic chemistry.
In terms of nomenclature, epoxides are often treated as their own functional group because of their distinctive properties. The term "epoxide" is widely used, while "oxerane" may appear in some academic texts. Both terms refer to the same structure, emphasizing the importance of recognizing these synonyms in chemical literature.
Understanding the reactivity of epoxides is crucial, as they can easily undergo ring-opening reactions, which are fundamental in various synthetic pathways. This reactivity is a key aspect of their utility in organic synthesis, allowing chemists to manipulate these compounds for diverse applications.