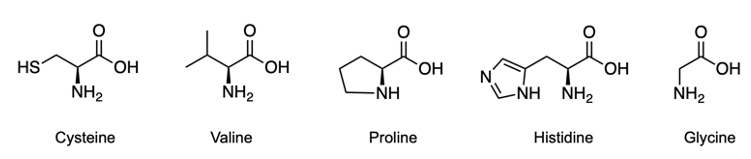
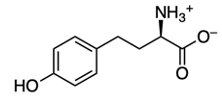
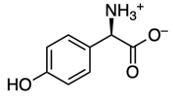
Amino acids are classified as carboxylic compounds that can be detected visually using Ninhydrin, an aromatic diketone hydrate. Ninhydrin reacts specifically with primary amines found in amino acids. Structurally, Ninhydrin features an aromatic portion, characterized by a benzene ring, and two carbonyl groups, which together form a diketone. Additionally, it exists as a geminal diol, meaning the hydroxyl groups are attached to the same carbon atom.
When Ninhydrin interacts with an amino acid, it produces a dye known as Ruhemann's purple. This reaction has practical applications, particularly in forensic science, where it is utilized to detect fingerprints on various surfaces. The amino acids present in human sweat react with Ninhydrin, allowing forensic experts to visualize fingerprints, often depicted in crime dramas.
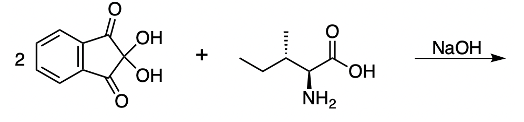
The reaction mechanism involves two moles of Ninhydrin reacting with one mole of an amino acid, establishing a 2:1 stoichiometric relationship. The presence of sodium hydroxide facilitates the formation of anhydrin, which is crucial for the reaction. The outcomes of this interaction include:
- Deamination: The amino group from the amino acid is incorporated into the dye.
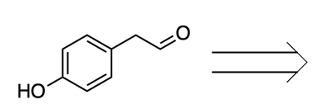
- Decarboxylation: The carboxyl group is released as carbon dioxide (CO2).
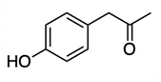
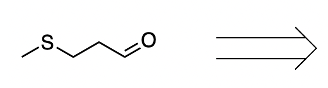
- Aldehyde Formation: The alpha carbon of the amino acid gains a double bond to oxygen, resulting in the formation of an aldehyde.
In summary, the Ninhydrin test is a vital analytical method that involves the reaction of two moles of Ninhydrin with one mole of an amino acid, yielding Ruhemann's purple dye, along with aldehyde and carbon dioxide as byproducts. This process highlights the significance of amino acids in biochemical reactions and their applications in real-world scenarios.