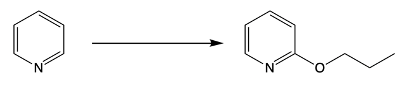
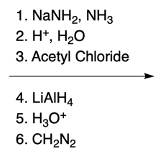
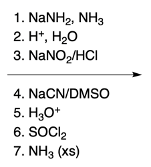
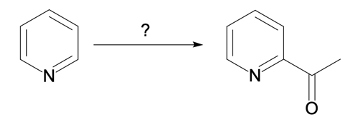
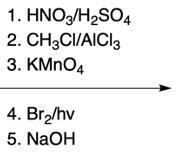
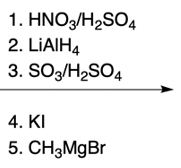
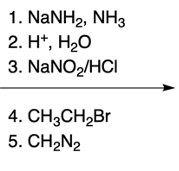
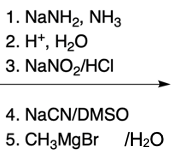
Nucleophilic aromatic substitution (SNAr) reactions are a key area of study in organic chemistry, particularly when examining compounds like pyridine. Pyridine's electron deficiency makes it more reactive towards nucleophilic substitution compared to benzene. Two significant SNAr reactions involving pyridine occur at the ortho position, with the Chi Chi Babin reaction being a notable example.
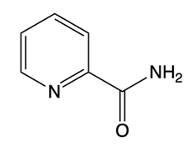
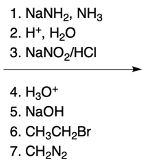
The Chi Chi Babin reaction is a method for synthesizing 2-aminopyridine by reacting pyridine with sodium amide, a strong base. In this reaction, the hydrogen atom at the ortho position of the pyridine ring is replaced by an amino group (NH2). The reaction proceeds in the presence of ammonia as a solvent, followed by acidic hydrolysis using H+ and water. The overall transformation can be summarized as follows:
Pyridine + Sodium Amide → 2-Aminopyridine + H2 (byproduct)
In this process, the hydrogen atom is substituted, resulting in the formation of 2-aminopyridine, while hydrogen gas is released as a byproduct. This reaction exemplifies the addition-elimination mechanism characteristic of nucleophilic aromatic substitutions.
Understanding the mechanism of this reaction is crucial, as it builds on previously learned concepts of nucleophilic aromatic substitution. The addition of the nucleophile (NH2) to the electron-deficient aromatic ring leads to the formation of an intermediate, which subsequently undergoes elimination to yield the final product, 2-aminopyridine.