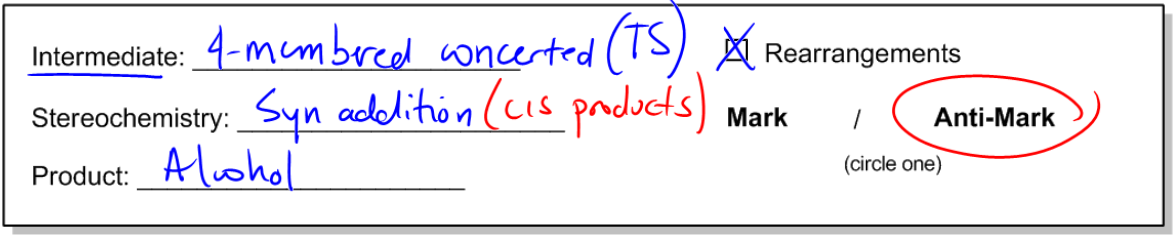
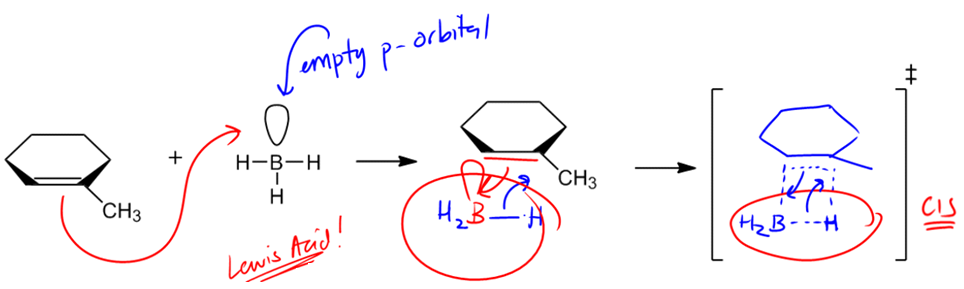
In organic chemistry, the addition of alcohols to double bonds can occur through various mechanisms, each with distinct characteristics. One notable reaction is hydroboration-oxidation, which introduces alcohol to double bonds in a unique manner. Unlike hydration and oxymercuration, which involve carbocation intermediates and follow Markovnikov's rule, hydroboration-oxidation utilizes a concerted mechanism that does not form a carbocation. Instead, it features a four-membered transition state, which is crucial for understanding the reaction's stereochemistry.
The stereochemistry of hydroboration-oxidation is defined by syn addition, meaning that the resulting products will be cis to each other. This contrasts with the anti addition seen in oxymercuration, where trans products are formed. The significance of syn addition lies in the fact that it allows for the formation of alcohols at the less substituted carbon atom, adhering to the anti-Markovnikov rule. This is particularly useful in synthetic applications, as it enables chemists to selectively add functional groups to less hindered positions on a molecule.
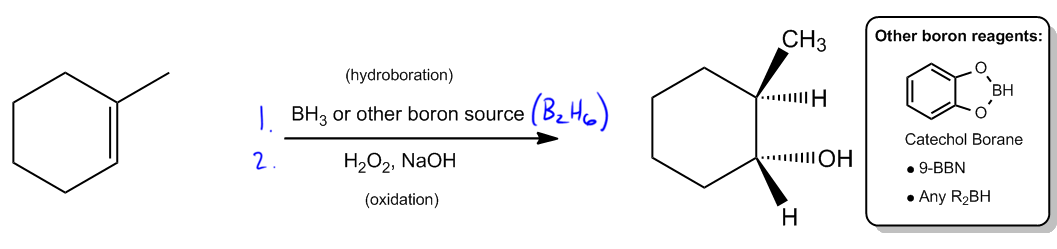
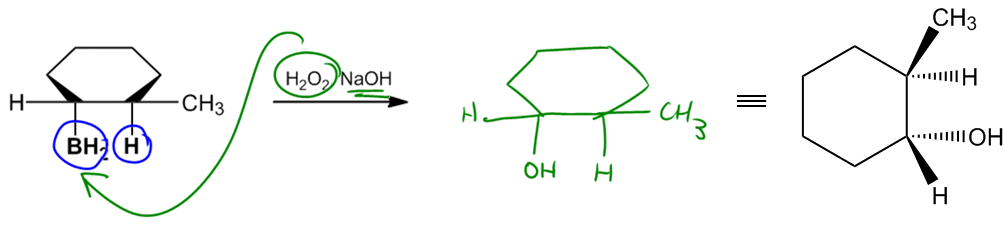
The hydroboration step typically involves the use of boron sources such as borane (BH3) or diborane (B2H6), both of which can serve as effective reagents. Other boron sources, like catecholborane or 9-borabicyclo[3.3.1]nonane (9-BBN), may also be employed depending on the specific context or instructor preferences. Following hydroboration, the oxidation step is carried out using hydrogen peroxide in a basic medium, converting the boron intermediate into an alcohol.
Ultimately, the product of hydroboration-oxidation is an alcohol that is anti-Markovnikov and exhibits syn stereochemistry. This means that the hydroxyl group (–OH) will be positioned at the less substituted carbon, and it will be cis to the hydrogen atom added during the reaction. Understanding this reaction mechanism is essential for mastering the addition of alcohols to alkenes and for applying these concepts in synthetic organic chemistry.