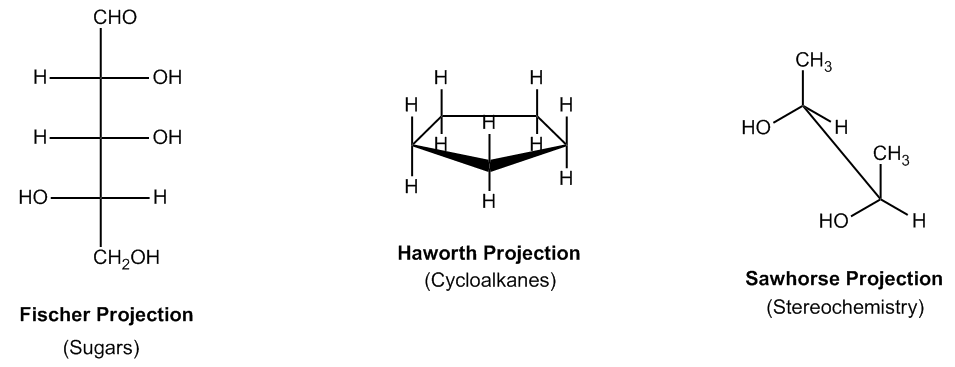
In organic chemistry, various projection methods are utilized to represent molecular structures, each serving specific purposes. One prominent type is the Fischer Projection, primarily used for sugars and carbohydrates. This projection allows chemists to visualize the arrangement of atoms in two dimensions, making it easier to analyze the stereochemistry of sugars.
Another important projection is the Haworth Projection, which depicts cyclic structures in a three-dimensional format. This method is particularly useful for illustrating the orientation of substituents at the top and bottom of a ring, providing clarity on the spatial arrangement of atoms in cyclic compounds.
The Sawhorse Projection is also significant, especially in the study of stereochemistry. It helps convey the relative orientation and configuration of atoms within a molecule, allowing for a better understanding of how these atoms interact in three-dimensional space.
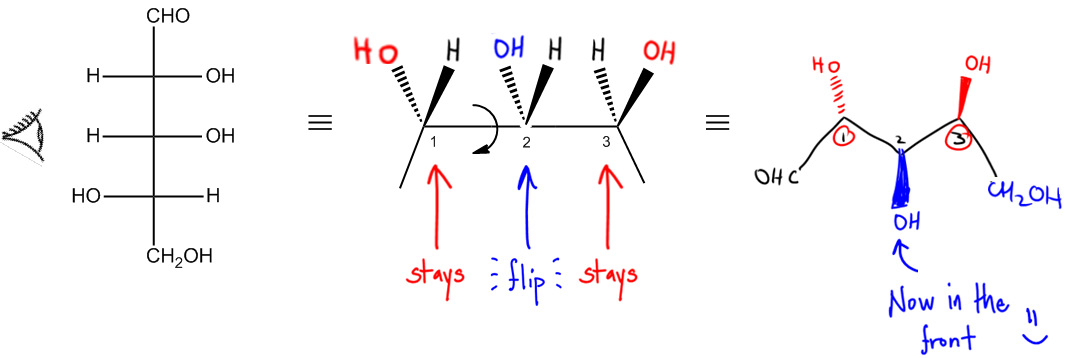
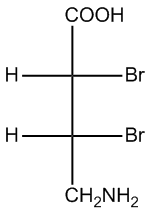
Regardless of the projection used—be it Fischer, Haworth, or Sawhorse—converting these representations into bond-line structures is essential for comprehensive analysis. Bond-line structures serve as a standardized metric for comparing different molecules, facilitating clearer communication and understanding in organic chemistry.