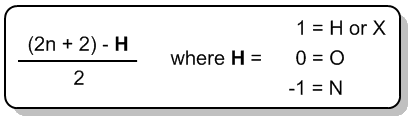
The index of hydrogen deficiency (IHD) is a crucial concept in organic chemistry that helps determine the saturation level of a molecule. Understanding IHD is particularly beneficial when studying constitutional isomers, as it simplifies the process of identifying different structural forms of a compound. The IHD indicates how many hydrogen atoms are missing from a molecule compared to a fully saturated structure, which is defined by the formula \(2n + 2\), where \(n\) represents the number of carbon atoms in the molecule. This formula allows chemists to predict the maximum number of hydrogen atoms a saturated molecule can contain.
A saturated molecule has the maximum number of hydrogen atoms possible, while an unsaturated molecule has fewer hydrogen atoms than predicted by the \(2n + 2\) rule. The terms "saturated" and "unsaturated" are also used in culinary contexts, such as with fats. Saturated fats contain long carbon chains with the maximum number of hydrogen atoms, whereas unsaturated fats are missing some hydrogen atoms due to the presence of double or triple bonds.
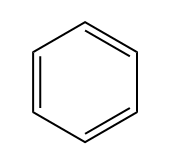
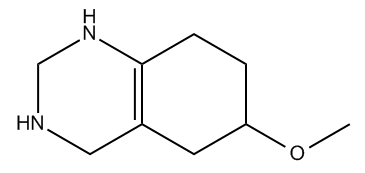
There are two primary reasons why a molecule may be unsaturated: the presence of pi bonds and the formation of rings. Pi bonds occur in double and triple bonds, which reduce the number of hydrogen atoms attached to the carbon atoms. Additionally, when carbon atoms form a ring structure, the ends that would typically be bonded to hydrogen atoms must connect, resulting in fewer hydrogen atoms overall. For example, in a ring, two terminal carbon atoms that would normally be \(CH_3\) become \(CH_2\) due to the fusion of the ends.
In summary, the index of hydrogen deficiency is a valuable tool for understanding the saturation of organic compounds, aiding in the identification and differentiation of isomers. By applying the \(2n + 2\) formula and recognizing the effects of pi bonds and ring structures, students can gain deeper insights into molecular structures and their properties.


 Degrees of Unsaturation general formula
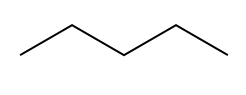
Degrees of Unsaturation general formula IHD hexane formula
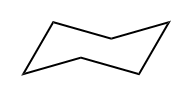
IHD hexane formula IHD cyclohexane formula
IHD cyclohexane formula IHD of hexane and cyclohexane
IHD of hexane and cyclohexane Molecular formula blank
Molecular formula blank Structure question blank
Structure question blank Molecular formula answered
Molecular formula answered Structure questions answered
Structure questions answered
