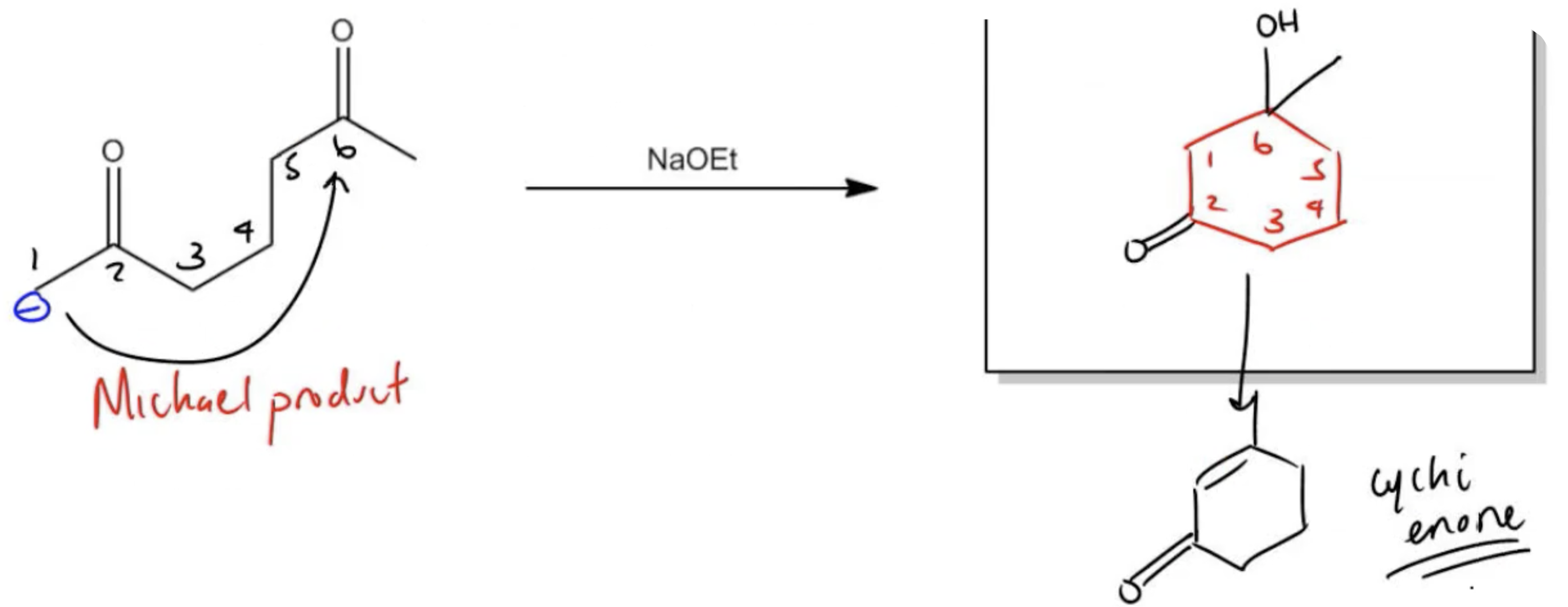
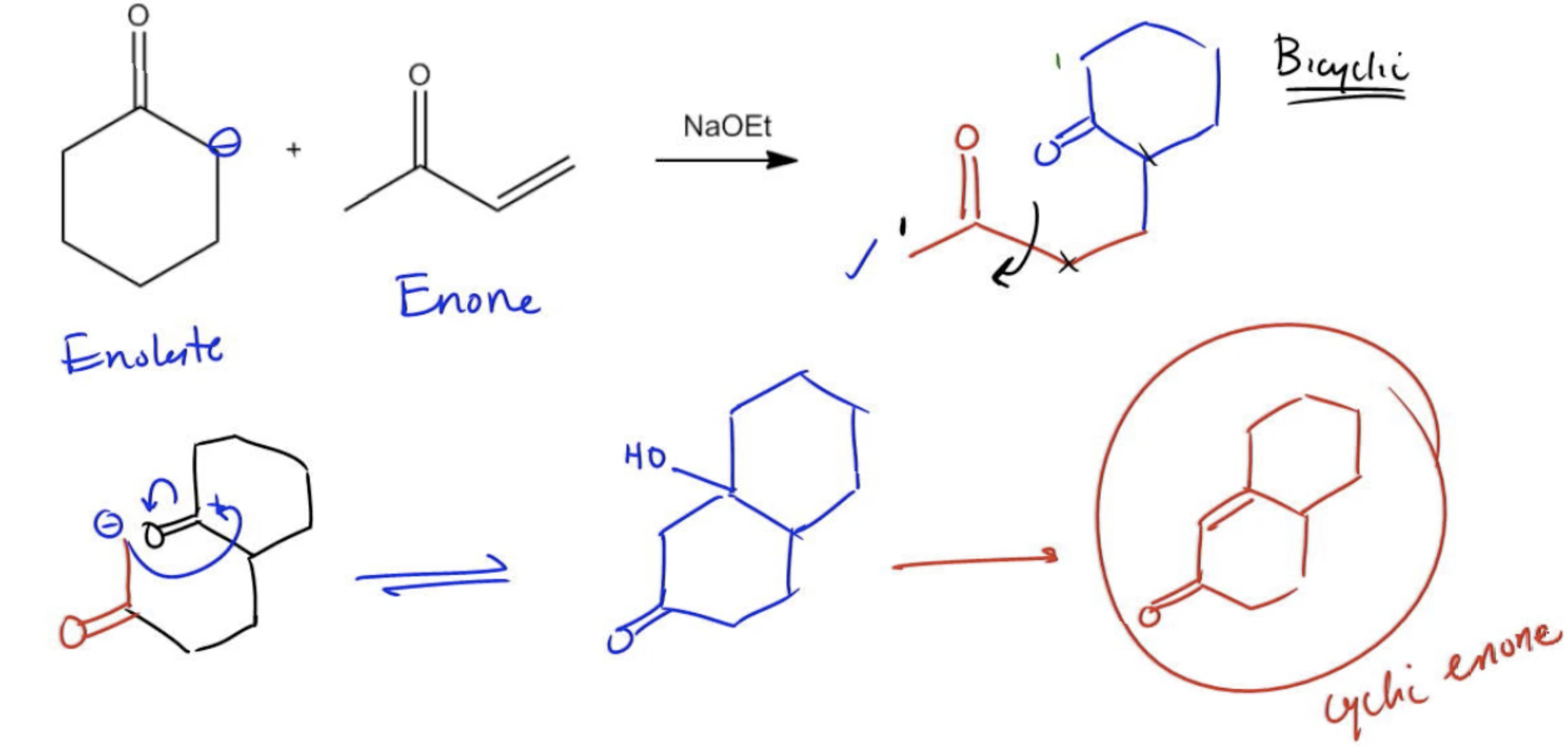
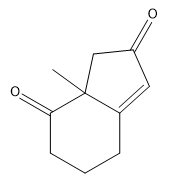
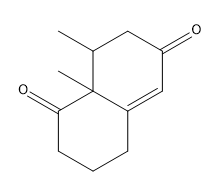
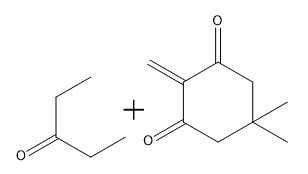
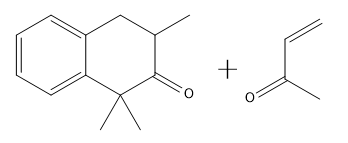
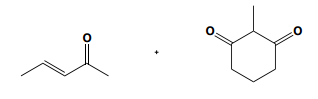In organic chemistry, the formation of 15 dicarbonyl compounds through intramolecular self-condensation leads to the creation of 6-membered enones. This process is enhanced by the Michael reaction, where an enone reacts with an enolate to generate a 15 dicarbonyl compound. The subsequent cyclization of this compound is known as the Robinson annulation, which can be conceptualized as a series of aldol reactions.
The Robinson annulation involves three key steps: first, an aldol reaction produces the initial enone, which is an α,β-unsaturated carbonyl compound. Next, a second aldol reaction occurs when the enolate attacks the enone via conjugate addition, resulting in the formation of the 15 dicarbonyl. Finally, this 15 dicarbonyl undergoes cyclization to yield a new 6-membered enone, completing the process.
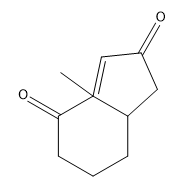
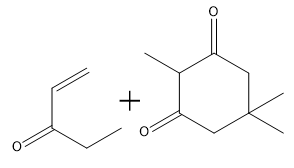
When drawing the product of a Robinson annulation, it is crucial to determine the correct position for the enolate attack. For instance, if the enolate is placed in a position that does not allow for the formation of a 6-membered ring, the reaction will yield a 4-membered ring instead. Therefore, careful consideration of the molecular structure is necessary to ensure the desired product is achieved.
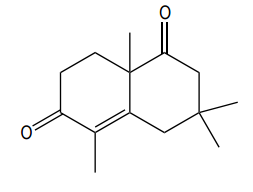
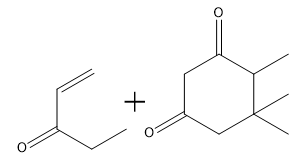
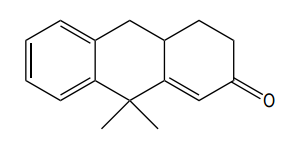
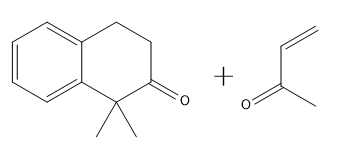
To illustrate this, consider a scenario where you start with a Michael reaction involving an enone and an enolate. After forming the Michael product, you would then proceed to perform the Robinson annulation. The final product typically features a cyclic enone, which may undergo dehydration to yield a more stable structure, often represented as a methyl-substituted double bond within the ring.
In summary, the Robinson annulation is a powerful synthetic strategy in organic chemistry that combines the principles of aldol reactions and conjugate addition to create complex cyclic structures from simpler precursors. Understanding the sequence of reactions and the importance of molecular positioning is essential for successful synthesis.