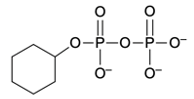
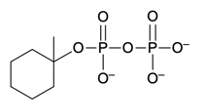
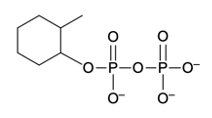
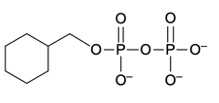
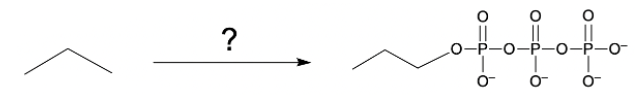
Phosphate anhydrides are compounds formed by the linkage of two or more phosphate groups. The simplest neutral form of a phosphate anhydride is pyrophosphoric acid, which is produced through a condensation reaction between two phosphoric acid molecules. In this reaction, a catalyst, typically H+, facilitates the loss of water, allowing the two phosphoric acid molecules to combine into a single molecule. The resulting structure retains hydroxyl (OH) groups, indicating its acidic form.
In biological systems, which are slightly basic (around pH 7.4), pyrophosphate predominates. In this environment, the hydroxyl groups become deprotonated, resulting in negatively charged oxygen atoms. This transformation is crucial as it highlights the role of pyrophosphate in biological processes.
One of the most significant examples of a phosphate anhydride is Adenosine Triphosphate (ATP). ATP contains a portion that is a phosphate anhydride, characterized by its negative oxygens due to the basic environment. The structure of ATP includes a ribose sugar and a nitrogenous base, adenine. When one phosphate group is lost from ATP, it forms Adenosine Diphosphate (ADP), which contains two phosphate groups. Understanding the formation and function of phosphate anhydrides, particularly ATP and ADP, is essential in biochemistry, as they play critical roles in energy transfer within cells.