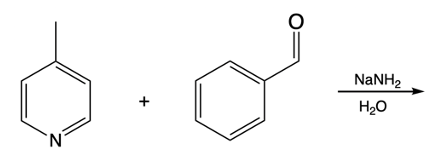
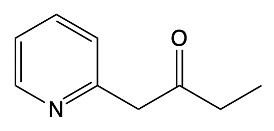
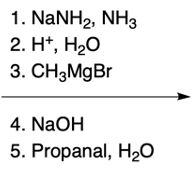
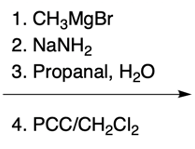
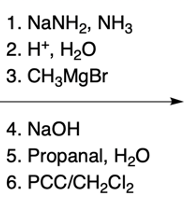
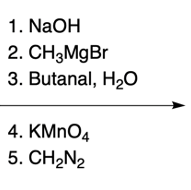
In the study of substituted pyridines, understanding the side chain reactions is crucial, particularly regarding the acidity of benzylic hydrogens. The benzylic position refers to the carbon atom directly connected to an aromatic ring, such as benzene or pyridine. Benzylic hydrogens are acidic due to the stability of the resulting carbanions, which are intermediates formed when a hydrogen ion (H+) is removed.
When comparing ordinary benzylic carbanions, which are stabilized by resonance, to pyridine carbanions, it becomes evident that pyridine carbanions exhibit even greater acidity. This increased acidity is attributed to two factors: resonance stabilization and the inductive effect of the electronegative nitrogen atom present in pyridine. The presence of nitrogen allows for better stabilization of the negative charge formed during deprotonation.
The pKa values of these carbanions illustrate this difference. For ordinary benzylic carbanions, the pKa is approximately 41, indicating a relatively low acidity. In contrast, pyridine carbanions have a pKa ranging from 34 to 36, signifying a higher acidity due to the influence of the nitrogen atom. A lower pKa value correlates with increased acidity, meaning that pyridine carbanions are more readily formed when a strong base deprotonates the benzylic carbon.
Strong bases, such as organolithiums and sodium amide, can effectively deprotonate the benzylic position, leading to the formation of various carbanions. The unique properties of pyridine, particularly the resonance and inductive effects from the nitrogen atom, make its carbanions particularly noteworthy in organic chemistry.