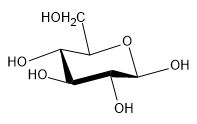
The cyclization of monosaccharides is a crucial reaction that occurs in aqueous solutions, where these sugars predominantly exist in cyclic forms. Monosaccharides can form stable five-membered rings, known as furanoses, and six-membered rings, referred to as pyranoses. Notably, four-membered and larger rings are generally unstable and not commonly encountered.
The cyclization process involves the nucleophilic addition of the penultimate alcohol to the electrophilic carbonyl carbon. This reaction typically results in the formation of two distinct cyclic structures due to the trigonal planar geometry of the carbonyl carbon, which allows for attack from either the top or bottom. This leads to the creation of two stereoisomers known as anomers, specifically at the C1 position. The two anomers are classified as the alpha (α) and beta (β) forms, based on the orientation of the hydroxyl group (OH) at the anomeric carbon. In the α-anomer, the OH group is positioned trans to the penultimate carbon (C5), while in the β-anomer, it is cis.
For D-glucose, the α-anomer has the OH group facing down, indicating that the nucleophilic attack occurred from the top, whereas the β-anomer has the OH group facing up, suggesting an attack from the bottom. The predominance of the β-anomer in solution (approximately 64% β and 36% α) can be attributed to the principle of equatorial preference, where larger groups prefer to occupy equatorial positions in cyclic structures.
As monosaccharides cyclize, their nomenclature changes. For instance, D-glucose becomes D-glucopyranose upon forming a six-membered ring. The designation of α or β is included in the name to indicate the specific anomer. Understanding the orientation of other hydroxyl groups in the cyclic form can be simplified using the mnemonic "downright." This rule states that if a hydroxyl group is on the right in the Fischer projection, it will point down in the ring, while those on the left will point up. The exception is the penultimate carbon, which, if it is a D-sugar, will always face up in the ring.
Additionally, the process of mutarotation describes the interconversion between the α and β anomers through the straight-chain form, allowing for the shuffling of the hydroxyl groups. This dynamic equilibrium is essential for understanding the behavior of monosaccharides in solution.