The haloform reaction is a specific type of base-catalyzed alpha halogenation that occurs with methyl ketones. This reaction begins with the formation of an enolate from the methyl ketone, which then undergoes halogenation at the alpha carbon. The key distinction in the haloform reaction is that the presence of a methyl group adjacent to the carbonyl allows for the formation of a highly reactive leaving group, CX3, after multiple halogenation steps.
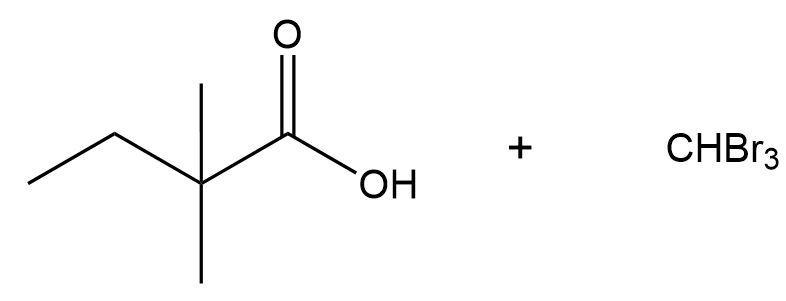
In the mechanism, after the enolate forms and reacts with halogen (X), a polyhalogenated alpha carbon is produced. The next equivalent of base can then facilitate an SN2 mechanism, leading to the formation of a tetrahedral intermediate represented as O-R-C(X)3. Here, the carbon with three halogens becomes an excellent leaving group, allowing the reaction to proceed further. The tetrahedral intermediate ultimately collapses, ejecting the CX3 group and yielding a carboxylic acid and a halide ion (X-).
Following this, the halide ion can deprotonate the carboxylic acid, resulting in the formation of a carboxylate ion. The product of the haloform reaction is a haloform compound, which is characterized by a methyl group where three hydrogens have been replaced by halogens. Depending on the halogen used, the resulting haloform can be chloroform (if chlorine is used) or iodoform (if iodine is used). The formation of iodoform, in particular, is significant in laboratory settings as it serves as a qualitative test for the presence of methyl ketones. The appearance of a yellow precipitate indicates the successful formation of iodoform, confirming the presence of a methyl group on the ketone.
This reaction highlights the importance of the leaving group in facilitating the transformation of the alpha carbon, which is a crucial aspect that distinguishes the haloform reaction from other base-catalyzed mechanisms.