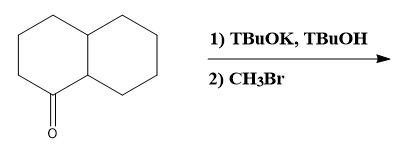
Enolate alkylations and acylations are straightforward reactions that involve the use of enolates, which are formed by deprotonating the alpha carbon of a carbonyl compound. The process begins when a base abstracts a proton from the alpha carbon, resulting in a negatively charged enolate ion. This enolate can then act as a nucleophile, attacking either an alkyl halide or an acid chloride.
When an enolate reacts with an alkyl halide, the outcome is an alpha-alkylated carbon compound. This reaction introduces an alkyl group to the carbon adjacent to the carbonyl, effectively modifying the structure of the original compound. Conversely, if the enolate reacts with an acid chloride, the result is an acylated carbon, leading to the formation of a beta-dicarbonyl compound. The product will include an R group that corresponds to the alkyl or acyl group introduced during the reaction.
Overall, understanding the mechanism of enolate alkylation and acylation is essential for manipulating carbonyl compounds in organic synthesis. The simplicity of these reactions lies in the ability to generate enolates and their subsequent nucleophilic attacks, making them valuable tools in the chemist's repertoire.