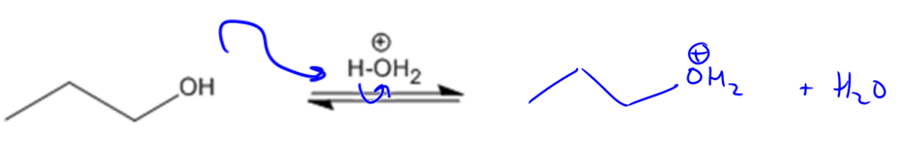
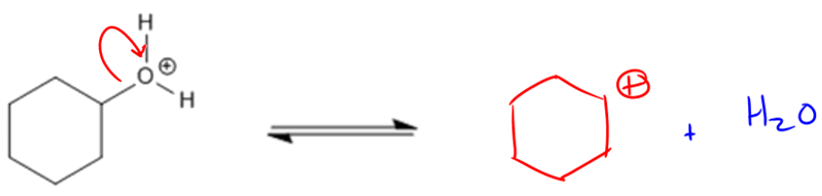
Acid-catalyzed dehydration is a chemical process that transforms alcohols into alkenes by removing water. This mechanism relies on the concept of leaving groups, which are species that can depart from a molecule, often forming a conjugate base. In the case of alcohols, the hydroxide ion (OH-) is a poor leaving group because it is a strong base. To enhance the leaving ability of the alcohol, an acid is introduced, which protonates the alcohol, converting it into water (H2O). Water is a much better leaving group due to its neutral charge, facilitating the elimination reaction.
The general reaction can be represented as follows: when an alcohol reacts with an acid, two sigma bonds are broken—one from the alcohol and one from a beta hydrogen (the hydrogen atom on the carbon adjacent to the carbon bearing the hydroxyl group)—resulting in the formation of a pi bond (the double bond). This process is classified as an elimination reaction, where two bonds are removed to create one new bond.
Conversely, acid-catalyzed hydration is the reverse process, where a double bond is converted back into an alcohol through the addition of water. This is an addition reaction, characterized by the formation of two new bonds from one existing bond. The distinction between these two reactions hinges on the starting material: dehydration begins with an alcohol, while hydration starts with an alkene.
When considering the ease of dehydration, the structure of the alcohol plays a crucial role. Tertiary alcohols are the most favorable for dehydration due to their ability to form stable carbocations, which are positively charged carbon species. The stability of carbocations follows a trend: tertiary > secondary > primary, with methyl alcohol being unsuitable for this reaction as it cannot form a carbocation. Thus, tertiary alcohols dehydrate more readily than secondary or primary alcohols, making them the preferred substrates in acid-catalyzed dehydration reactions.