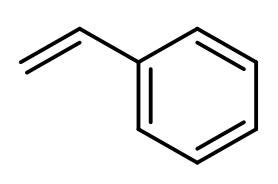
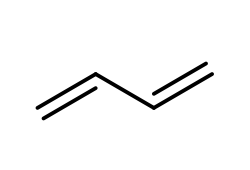
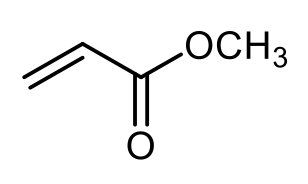
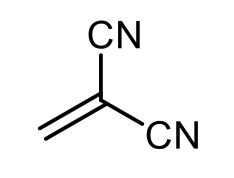
Anionic polymerization is a process that involves the polymerization of alkenes featuring electron-withdrawing groups. In this reaction, a strong nucleophile, commonly sodium amide or butyl lithium, acts as the initiator. The presence of the electron-withdrawing group on the alkene is crucial, as it enhances the reactivity of the alkene, allowing the nucleophile to effectively initiate the polymerization process.
During anionic polymerization, the nucleophile attacks the alkene, leading to the formation of a reactive anion. This anion then propagates the reaction by adding to additional alkene monomers, resulting in a long chain polymer. The repeating unit of the polymer is derived from the original alkene, although the exact number of repeating units is not specified.
In summary, when studying anionic polymerization, focus on identifying alkenes with electron-withdrawing groups, as they are essential for initiating the polymerization process. Understanding the role of strong nucleophiles in this context is also key to grasping the overall mechanism of anionic polymerization.