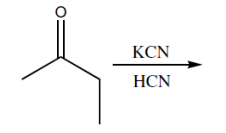
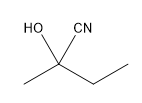
Nucleophilic addition is a fundamental reaction mechanism in organic chemistry, particularly when it comes to carbonyl compounds. One notable nucleophile that participates in this process is cyanide (CN-), which is commonly encountered in the formation of cyanohydrins. Cyanohydrins are functional groups characterized by the presence of both a cyanide group and a hydroxyl group (OH) on the same carbon atom.
The reaction typically begins with the nucleophilic attack of the cyanide ion on the carbonyl carbon, which possesses a strong partial positive charge. This results in the formation of a negatively charged alkoxide intermediate, where the cyanide is now bonded to the carbon. The next step involves protonation of the alkoxide, which can be achieved using water or a mild acid, leading to the formation of the cyanohydrin functional group.
Interestingly, cyanide can also be introduced into reactions using hydrogen cyanide (HCN). HCN is a weak acid with a pKa of approximately 10, meaning it can exist in an ionized form in aqueous solutions. This ionization allows HCN to provide both the cyanide nucleophile and a proton for the protonation step in a single reaction, simplifying the process compared to using sodium cyanide (NaCN) followed by a separate protonation step.
Once cyanohydrins are formed, they can undergo further reactions that are important to understand. These reactions are not directly related to the nucleophilic addition mechanism but are significant for the functional group chemistry of cyanohydrins. Exploring these subsequent reactions will enhance your understanding of the versatility and reactivity of cyanohydrins in organic synthesis.