Give the IUPAC names of the following compounds.
(a) CH3CH2C≡CCOOH
(b) CH3CH(NH2)CH(OH)COOH
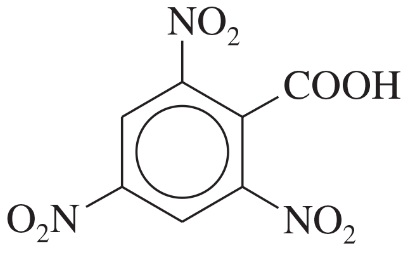
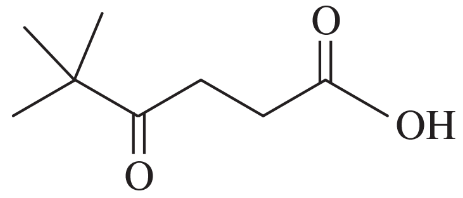
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 4:20m
4:20mMaster Carboxylic Acids Nomenclature with a bite sized video explanation from Johnny
Start learning