Textbook Question
Without referring to the chapter, draw the chair conformations of
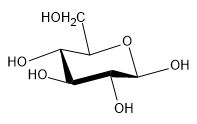
(c) β-D-galactopyranose (the C4 epimer of glucose).




 Verified step by step guidance
Verified step by step guidance
 12:58m
12:58mMaster Monosaccharides - Cyclization with a bite sized video explanation from Johnny
Start learning
