Multiple Choice
What molecule is removed from glucose molecules during the formation of maltose through glycosidic bond formation?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: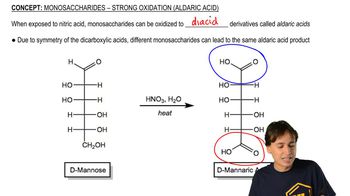
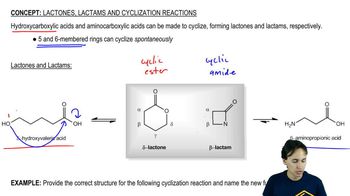
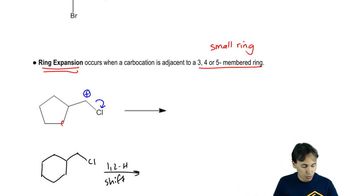

 12:58m
12:58mMaster Monosaccharides - Cyclization with a bite sized video explanation from Johnny
Start learning