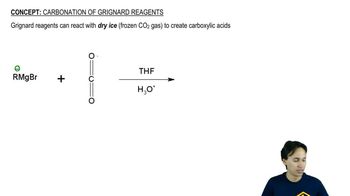
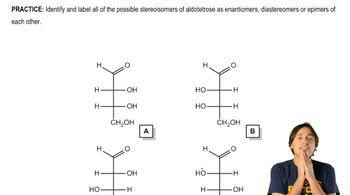
Textbook Question
Draw the α- and β-anomers of d-talopyranose. [The structure of talose is in Figure 27.11.]
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 12:58m
12:58mMaster Monosaccharides - Cyclization with a bite sized video explanation from Johnny
Start learning