Give the stereochemical relationships between each pair of structures. Examples are same compound, structural isomers, enantiomers, and diastereomers. Which pairs could you (theoretically) separate by distillation or recrystallization?
(a)
(b)
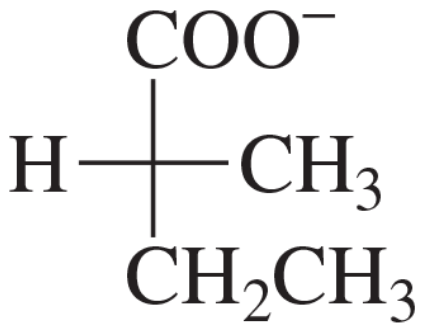
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 1:15m
1:15mMaster Introduction to different projections. with a bite sized video explanation from Johnny
Start learning