Textbook Question
a. There are 18 isomeric alkanes of molecular formula C8H18. Draw and name any eight of them.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: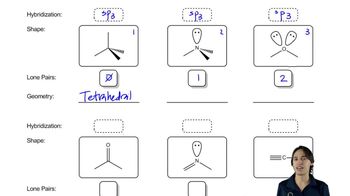
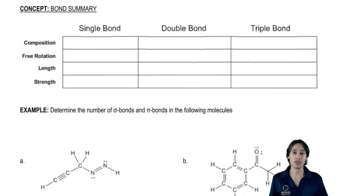
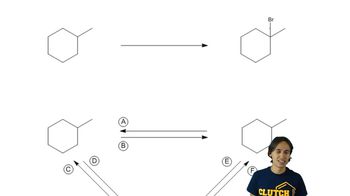

 3:43m
3:43mMaster The different parts of an IUPAC name with a bite sized video explanation from Johnny
Start learning