Give each substituent on the ten-carbon chain a common name and a parenthetical name
c.
d.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: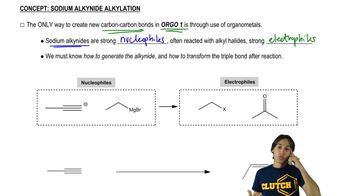
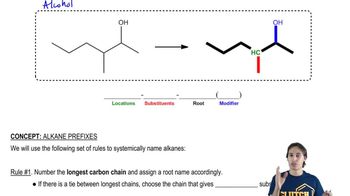


 6:42m
6:42mMaster Understanding Non-IUPAC Substituents with a bite sized video explanation from Johnny
Start learning