Textbook Question
Make a model of cyclooctatetraene in the tub conformation. Draw this conformation, and estimate the angle between the p orbitals of adjacent pi bonds.
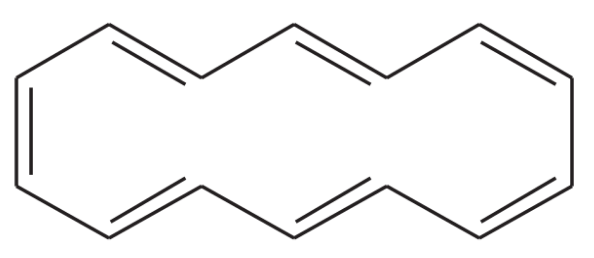
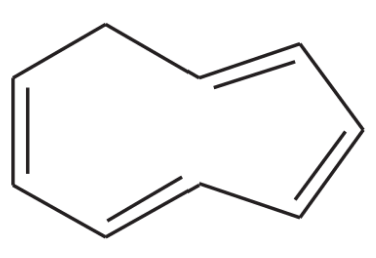
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:28m
4:28mMaster [6]annulene vs. [8]annulene with a bite sized video explanation from Johnny
Start learning