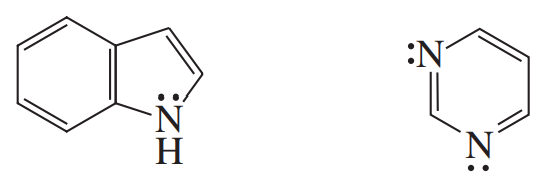
Consider the following compound, which has been synthesized and characterized:
a. Assuming this molecule is entirely conjugated, do you expect it to be aromatic, antiaromatic, or nonaromatic?
b. Why was this molecule synthesized with three tert-butyl substituents? Why not make the unsubstituted compound and study it instead?