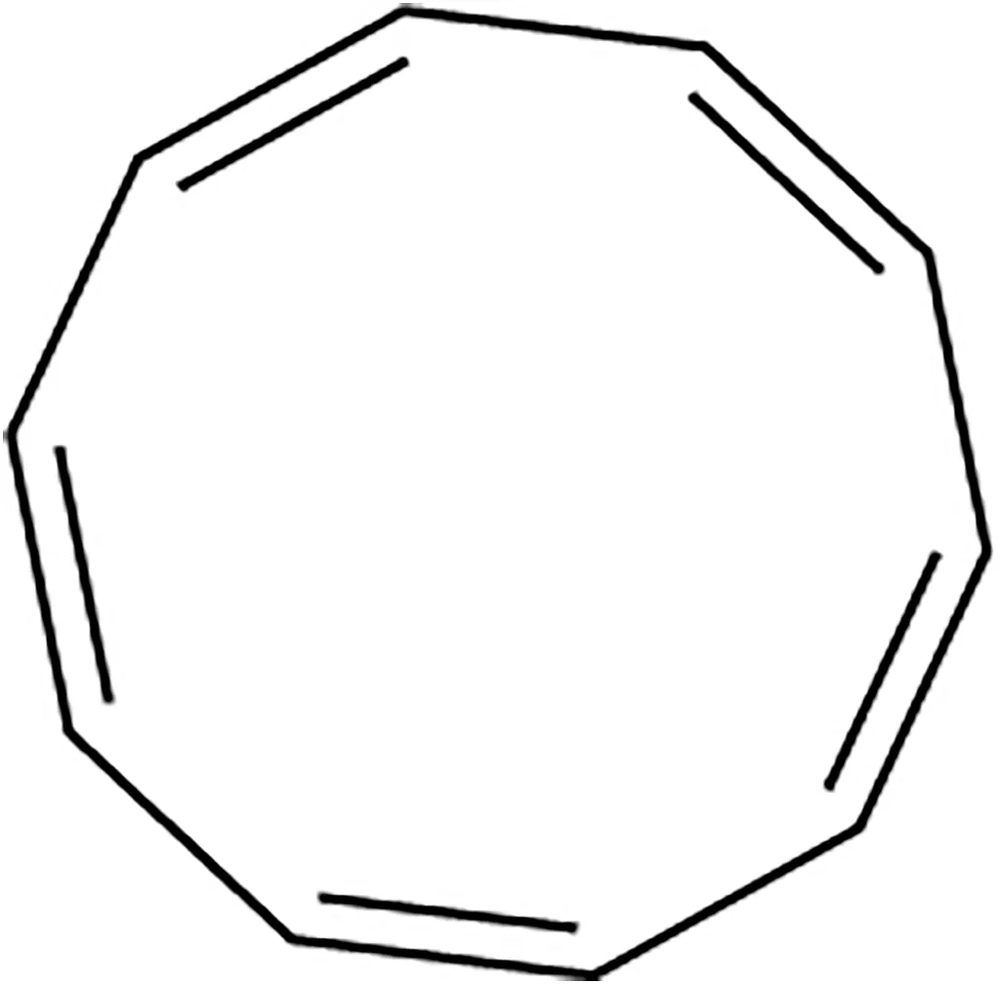
Textbook Question
Explain why each compound or ion should be aromatic, antiaromatic, or nonaromatic.
(a)
(b)
(c)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:28m
4:28mMaster [6]annulene vs. [8]annulene with a bite sized video explanation from Johnny
Start learning