Textbook Question
Classify the following compounds as aromatic, antiaromatic, or nonaromatic.
(a)
(b)
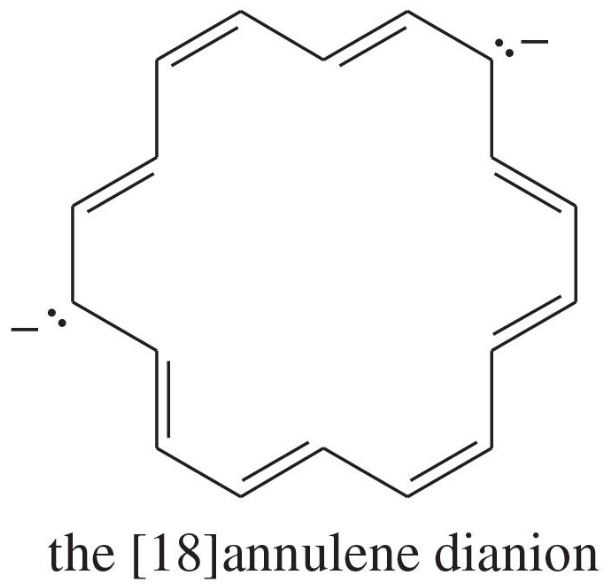
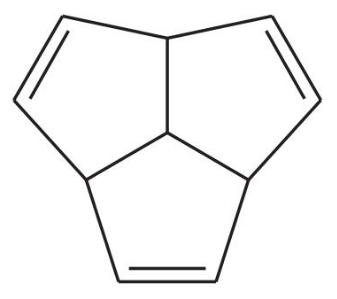
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
 4:28m
4:28mMaster [6]annulene vs. [8]annulene with a bite sized video explanation from Johnny
Start learning