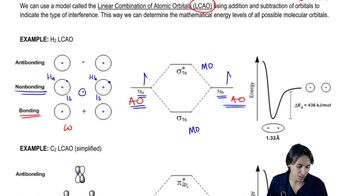
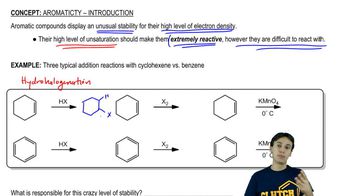
Textbook Question
Despite having only sp2-hybridized carbons and having 10 electrons (4n + 2) , the annulene shown is not aromatic. Why?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 4:28m
4:28mMaster [6]annulene vs. [8]annulene with a bite sized video explanation from Johnny
Start learning