Textbook Question
In an aqueous solution, D-glucose exists in equilibrium with two six-membered ring compounds. Draw the structures of these compounds.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: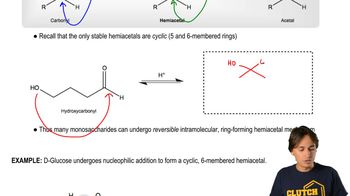
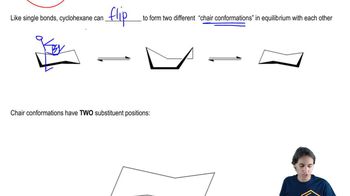
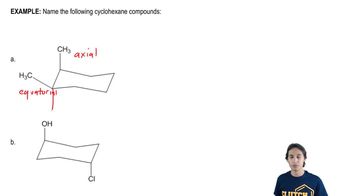

 12:58m
12:58mMaster Monosaccharides - Cyclization with a bite sized video explanation from Johnny
Start learning