The mass spectrum for a compound with molecular weight of 102 is shown below. Its IR spectrum has a broad, strong absorption at 3600 cm–1 and a medium absorption at 1360 cm–1.
b. Show the mechanism for formation of the peak at m/z = 84.
<IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: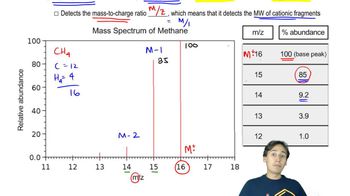


 4:28m
4:28mMaster Ionization Potentials with a bite sized video explanation from Johnny
Start learning