Draw complete Lewis structures for the following condensed structural formulas.
(d) CH2CHCHO
(e) (CH3)3CCOCHCH2
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: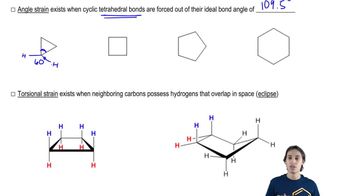


 6:06m
6:06mMaster How to interpret condensed structures. with a bite sized video explanation from Johnny
Start learning