Multiple Choice
Which of the following is the correct name of the following compound:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
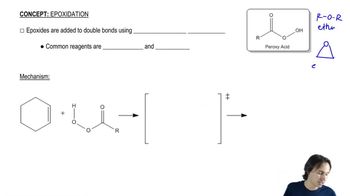

 1:09m
1:09mMaster Defining what an epoxide (oxirane) is. with a bite sized video explanation from Johnny
Start learning