Predict the products formed when CH3CH2–C≡C:–Na+ reacts with the following compounds.
(d) cyclohexanone
(e) CH3CH2CH2CHO
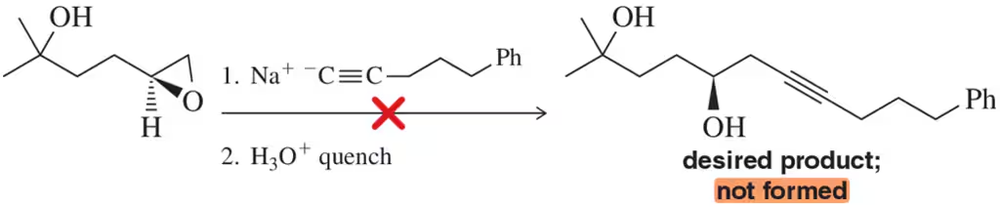
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 3:19m
3:19mMaster Sodium Alkynide Alkylation with a bite sized video explanation from Johnny
Start learning