When doing synthesis, you will often find yourself repeating the same series of steps. To see this in action, synthesize the following aldehydes beginning with an organic molecule containing three carbons or fewer.
(d)
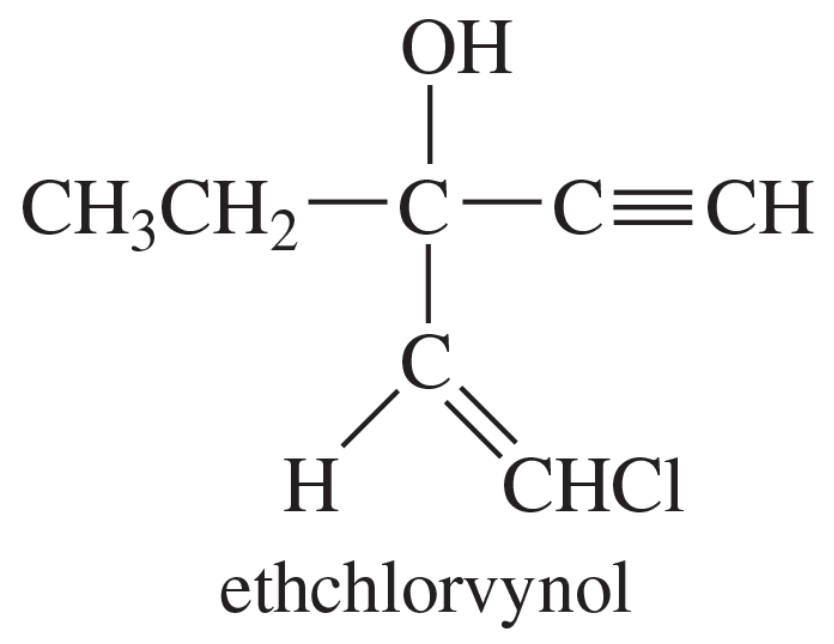
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:19m
3:19mMaster Sodium Alkynide Alkylation with a bite sized video explanation from Johnny
Start learning