LDA can be used to form enolates on esters and nitriles. Predict the product of these alkylation reactions.
(a)
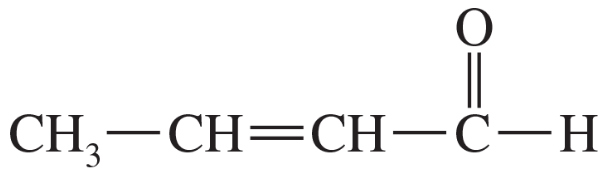
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:26m
2:26mMaster Formation of Enolates with a bite sized video explanation from Johnny
Start learning