Identify the enolate(s) that would form on treatment of each of the following carbonyls with base. [When there are two possibilities, draw both.]
(a)
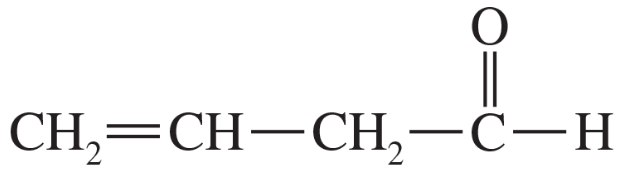
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:26m
2:26mMaster Formation of Enolates with a bite sized video explanation from Johnny
Start learning