For each molecule shown below,
1. indicate the most acidic hydrogens.
2. draw the important resonance contributors of the anion that results from removal of the most acidic hydrogen.
(g)
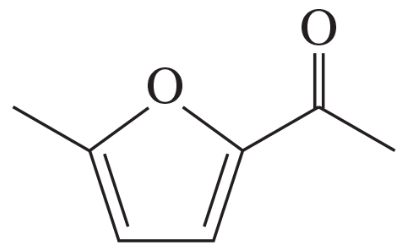
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:26m
2:26mMaster Formation of Enolates with a bite sized video explanation from Johnny
Start learning