For each molecule shown below,
2. draw the important resonance contributors of the anion that results from removal of the most acidic hydrogen.
(a)
(b)
(c)
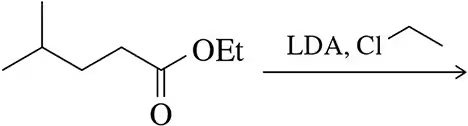

 2:26m
2:26mMaster Formation of Enolates with a bite sized video explanation from Johnny
Start learning