Name the following alkenes, being sure to specify whether they are cis or trans.
(c)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: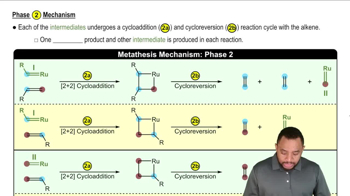

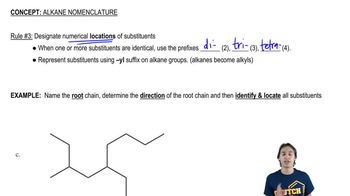

 4:28m
4:28mMaster How to name different types of double bonds or rings with a bite sized video explanation from Johnny
Start learning