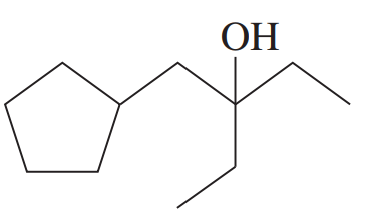
Propose a mechanism involving a hydride shift or an alkyl shift for each solvolysis reaction. Explain how each rearrangement forms a more stable intermediate.
Hint: Most rearrangements convert 2° (or incipient 1°) carbocations to 3° or resonance-stabilized carbocations.
(c)