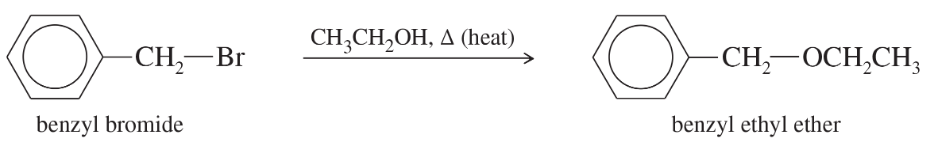
Predict the compound in each pair that will undergo solvolysis (in aqueous ethanol) more rapidly.
(c)
(d)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 1:49m
1:49mMaster Drawing the SN1 Mechanism with a bite sized video explanation from Johnny
Start learning