Allylic halides have the structure
a. Show how the first-order ionization of an allylic halide leads to a resonance-stabilized cation.
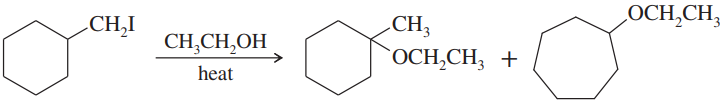
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
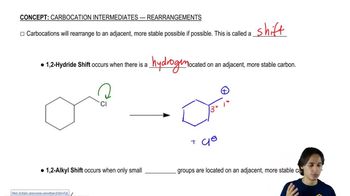

 1:49m
1:49mMaster Drawing the SN1 Mechanism with a bite sized video explanation from Johnny
Start learning