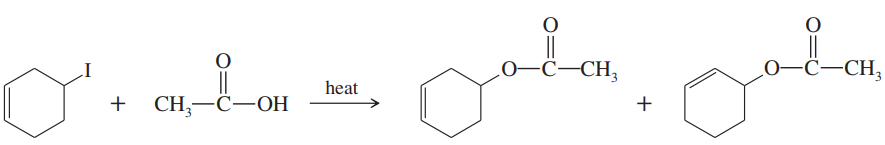
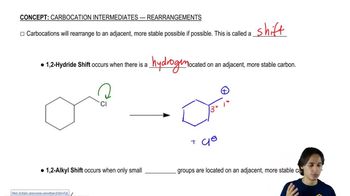
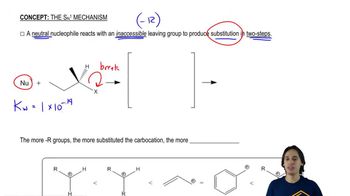
Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (i) SOCl₂ ; (ii) PBr₃ ; (iii) SOCl₂ , NEt₃ (iv) 1. TsCl, Et₃N 2. NaCN; (v) 1. TsCl, Et₃N 2. NaOt-Bu (vi) H₂SO₄ (vii) HCl; (viii) HBr; (ix) PCC; (x) H₂CrO₄ , H₂O (xi) HOCl, H₂O (xii) HIO₄ If no reaction occurs, write 'no reaction.'
(f)