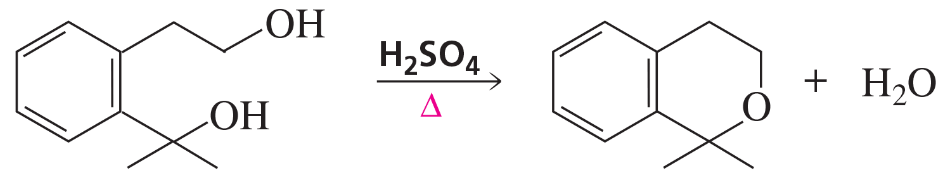
1,4-Dioxane is made commercially by the acid-catalyzed condensation of an alcohol.
(a) Show what alcohol will undergo condensation, with loss of water, to give 1,4-dioxane.
(b) Propose a mechanism for this reaction.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:33m
4:33mMaster The Mechanism of Alcohol Condensation. with a bite sized video explanation from Johnny
Start learning