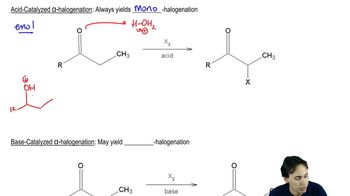
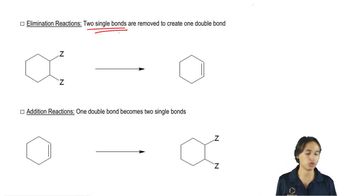
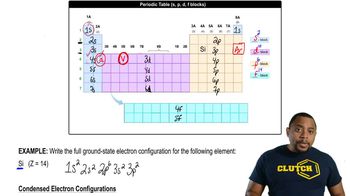
Which of the following ethers can be formed in good yield by condensation of the corresponding alcohols? For those that cannot be formed by condensation, suggest an alternative method that will work.
(a) dibutyl ether
(b) ethyl n-propyl ether
(c) di-sec-butyl ether