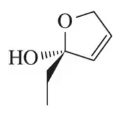
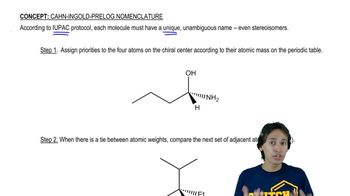
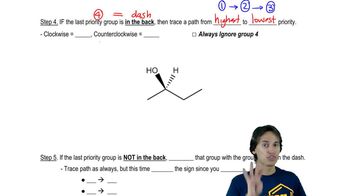
In Chapter 12, we introduce the SN2 reaction, a nucleophilic substitution reaction that proceeds with inversion. Confirm that inversion has occurred in each of the following examples by determining the absolute configuration of the chiral center in the reactants and products.
(a)