Complete the structure of each of these so that it matches the (R) or (S) configuration associated with the name.
(d)
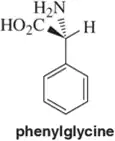
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:48m
1:48mMaster Why stereoisomers need their own naming system. with a bite sized video explanation from Johnny
Start learning