Assign the absolute stereochemistry for each of the following molecules.
(f)
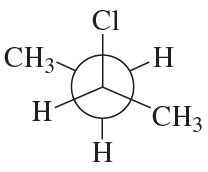
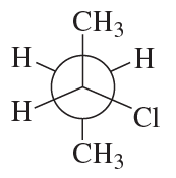
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
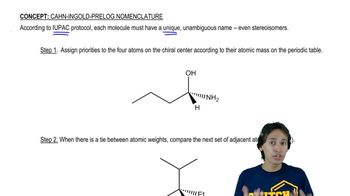
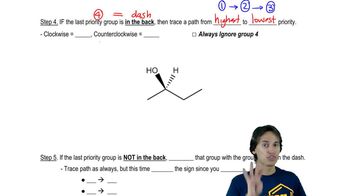

 1:48m
1:48mMaster Why stereoisomers need their own naming system. with a bite sized video explanation from Johnny
Start learning